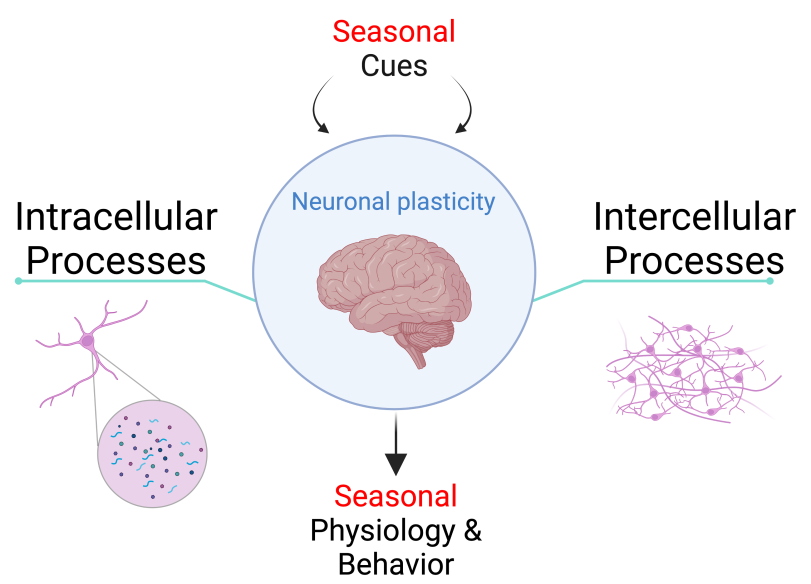
Our Overall Question
The human brain plastically changes across seasons. How is this process regulated? How is it orchestrated across the entire organ? What are the components driving this process? These are some of the questions our lab wants to address.
Integration of seasonal cues by the circadian clock
Organisms adapt to seasonal changes in photoperiod and temperature to survive; however, the mechanisms by which these signals are integrated in the brain are poorly understood. We are interested in understanding how changes in the molecular clock, in particular splicing, are triggered by seasonal cues and how this clock rearrangement participates in calendar timekeeping. We are also investigating how the clock conveys this information to endocrine centers through the expression and action of circadian neuropeptides.Selected publications:
- Hidalgo, S., Anguiano, M., Tabuloc, C. A., & Chiu, J. C. (2023). Seasonal cues act through the circadian clock and pigment-dispersing factor to control EYES ABSENT and downstream physiological changes. Current biology, 33(4), 675–687.e5. doi:10.1016/j.cub.2023.01.006
- Hidalgo, S., & Chiu, J. C. (2023). Integration of photoperiodic and temperature cues by the circadian clock to regulate insect seasonal adaptations. Journal of Comparative Physiology A, 10.1007/s00359-023-01667-1. doi:10.1007/s00359-023-01667-1(Review Article)
- Hidalgo, S., & Chiu, J. C. (2022). Glutamate and clock help bean bugs track seasonal reproductive changes. PLoS Biology, 20(9), e3001796. doi:10.1371/journal.pbio.3001796(Primer article)
- Cai, Y. D., Liu, X., Chow, G. K., Hidalgo, S., Jackson, K. C., Vasquez, C. D., Gao, Z. Y., Lam, V. H., Tabuloc, C. A., Zheng, H., Zhao, C., & Chiu, J. C. (2024). Alternative splicing of Clock transcript mediates the response of circadian clocks to temperature changes. Proc. Natl. Acad. Sci. U.S.A. 121 (50) e2410680121. 10.1073/pnas.2410680121. Previously published in bioRxiv doi:10.1101/2024.05.10.593646
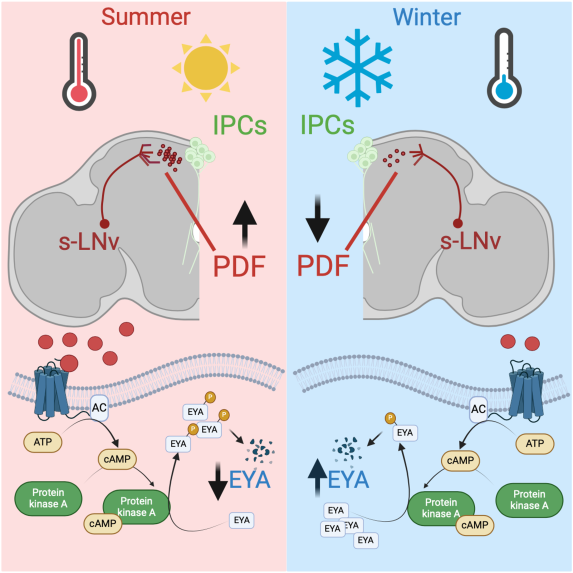
From Hidalgo et al., Current Biology, 2023
Seasonal plasticity of neuronal identity
One of the most striking characteristics of the changes the brain undergoes across seasons is the re-arrangement of neurotransmitters and neuropeptide release. Something that we once thought was static and that usually gives neurons their identity, the molecules they release, is now known to be dynamically defined. The environment has been described as a main driver for this change. Our lab is focused on understanding if this process, observed in several animal models, is also present in flies and other insects. For this, we are currently developing and deploying the next generation of genetically encoded sensors for neurotransmitters, such as dopamine and serotonin, and neuropeptides. In combination with electrochemical recordings and imaging tools, we look to describe the effect of temperature and photoperiods on neurotransmitter and neuropeptide release.Selected publications:
- Hidalgo, S., Fuenzalida-Uribe, N., Molina-Mateo, D., Escobar, A. P., Oliva, C., España, R. A., Andrés, M. E., & Campusano, J. M. (2021). Study of the release of endogenous amines in Drosophila brain in vivo in response to stimuli linked to aversive olfactory conditioning. Journal of Neurochemistry, 156(3), 337–351. doi:10.1111/jnc.15109
- Fuenzalida-Uribe, N., Hidalgo, S., Varas, R., Campusano, J.M. (2016). Study of the contribution of nicotinic receptors to the release of endogenous biogenic amines in Drosophila Brain. In: Li, M. (eds) Nicotinic Acetylcholine Receptor Technologies. Neuromethods, vol 117. Humana Press, New York, NY. 10.1007/978-1-4939-3768-4_4
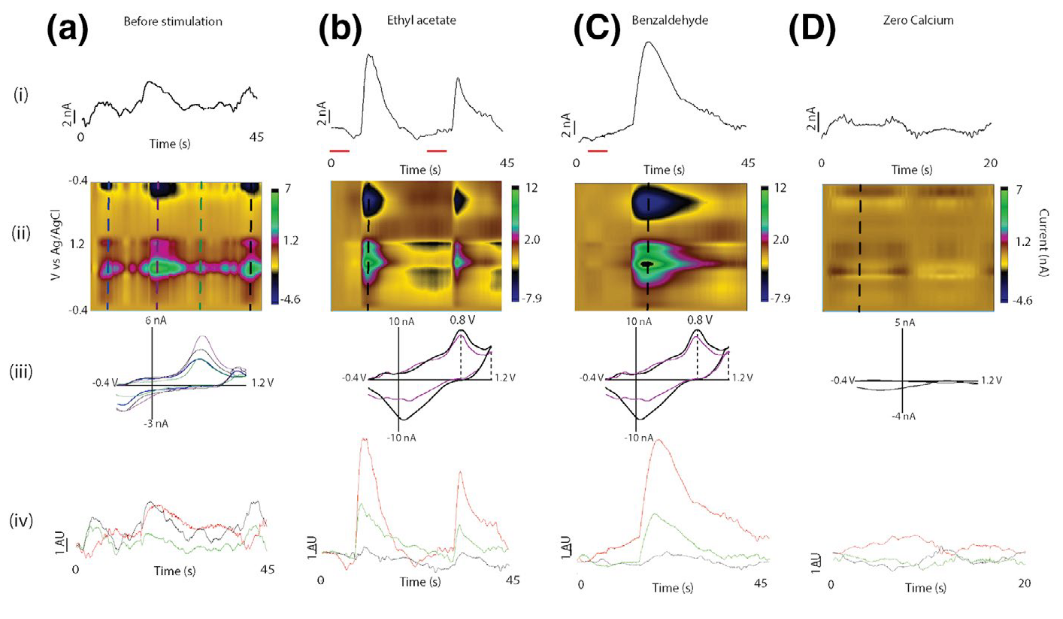
From Hidalgo et al., Journal of Neuroschemistry, 2021
Modelling seasonal disorders in flies
Parkinson's, Alzheimer's, depression, and schizophrenia are among the many disorders that show seasonal changes in their symptomatology. This not only affects the quality of life of patients but also affects treatment effectiveness and clinical diagnosis. In our lab, we use Drosophila to model these disorders to better understand the relationship between seasonal cues and disorder symptomatology and pathophysiology.Selected publications:
- Hidalgo, S., Campusano, J.M. & Hodge, J.J.L. (2021). Assessing olfactory, memory, social and circadian phenotypes associated with schizophrenia in a genetic model based on Rim. Translational Psychiatry, 11, 292. doi:10.1038/s41398-021-01418-3
- Hidalgo, S., Campusano, J. M., & Hodge, J. (2021). The Drosophila ortholog of the schizophrenia-associated CACNA1A and CACNA1B voltage-gated calcium channels regulate memory, sleep and circadian rhythms. Neurobiology of disease, 155, 105394. doi:10.1016/j.nbd.2021.105394
- Hidalgo, S., Castro, C., Zárate, R. V., Valderrama, B. P., Hodge, J.*, & Campusano, J. M. (2020). The behavioral and neurochemical characterization of a Drosophila dysbindin mutant supports the contribution of serotonin to schizophrenia negative symptoms. Neurochemistry International, 138, 104753. doi:10.1016/j.neuint.2020.104753
- Higham, J. P., Hidalgo, S., Buhl, E., & Hodge, J. (2019). Restoration of olfactory memory in Drosophila overexpressing human Alzheimer's disease associated Tau by manipulation of L-Type Ca2+ Channels. Frontiers in Cellular Neuroscience, 13, 409. doi:10.3389/fncel.2019.00409
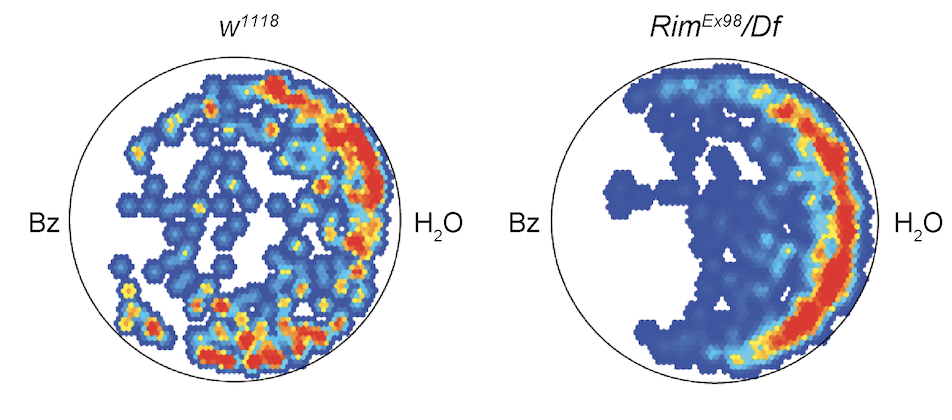
From Hidalgo et al., Translational Psychiatry, 2021
Funding

